Category: Author: Amy Pallant
Every education project funded by the National Science Foundation (NSF) is subject to flexible yet high expectations for the proposed research. NSF is investing in our ability to develop new technology, new curriculum, and new research that contributes innovative ideas and products to further the field of STEM education. When we are awarded an NSF […]
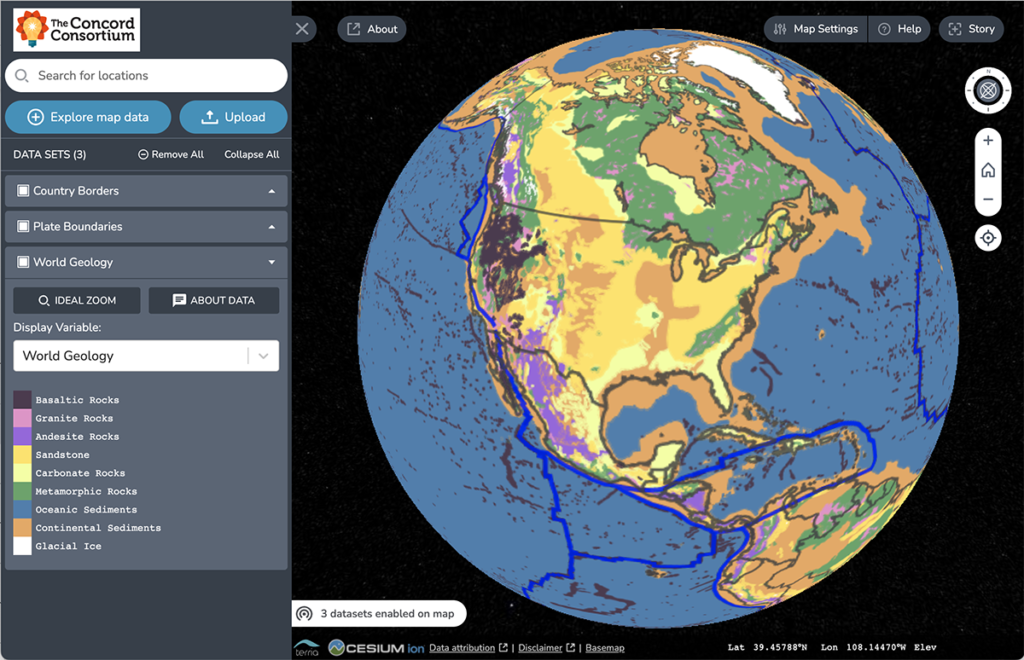
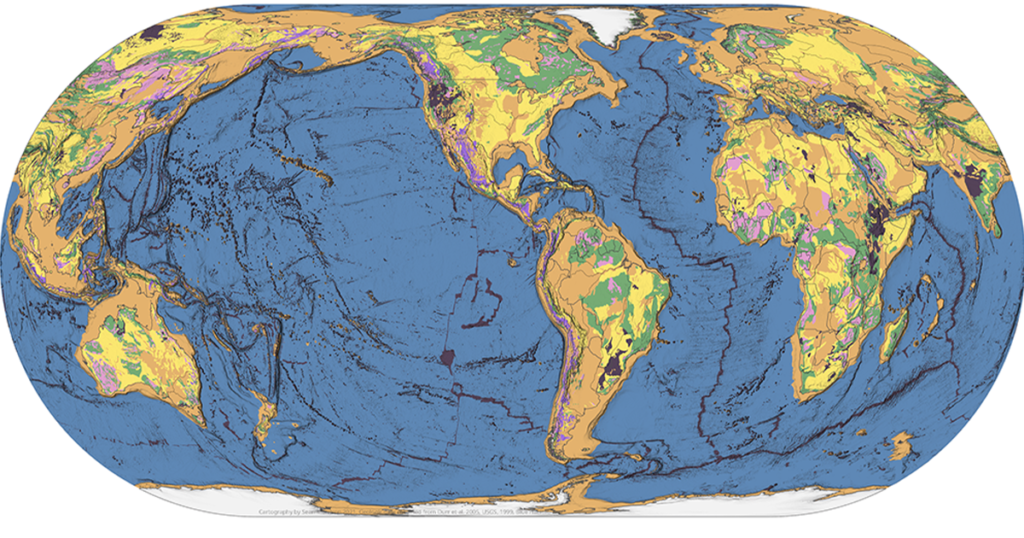
Traditional geologic maps beautifully illustrate the many different types of rock found on Earth’s surface. Geoscientists can look at a colorful geologic map and immediately spot important pieces of the story of Earth’s geologic history. For instance, in the map below, the red area found in Canada represents bedrock formed in the Late Archean Era […]
Is there a map that shows the distribution of the three major rocks types—igneous, metamorphic, and sedimentary—around the world? Our TecRocks team asked this question while trying to find real-world phenomena to engage students in the exploration of environments related to rock genesis. It seemed like a map like this should exist. Our hope was […]
Robert Constantinescu, a Ph.D. candidate in volcanology at the University of South Florida (USF), flew to the island of St. Vincent after the explosive activity at the La Soufrière volcano subsided. Between May 1 and 13, 2021, he worked alongside local scientists from the Seismic Research Centre of the University of West Indies to collect […]
The rock cycle, presented in nearly every Earth science curriculum and textbook, is typically taught in the same way from one classroom to the next. Students are shown an image that summarizes how all rocks are related to each other with suggested pathways by which one rock can transform into another rock. A nice, simple […]
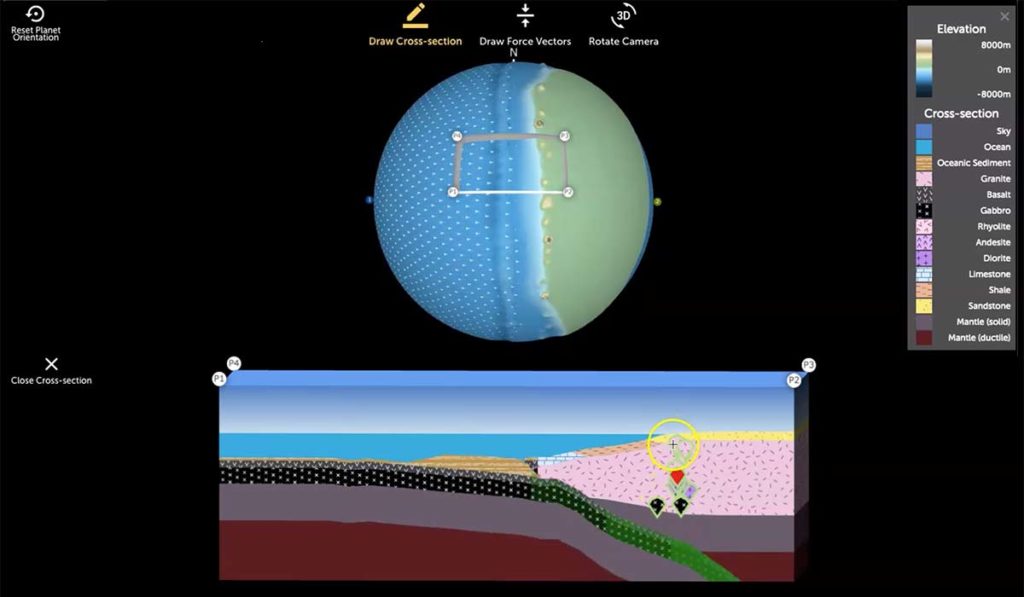
Earth science classes typically present plate tectonics and the rock cycle as separate and unrelated concepts. Yet land and rock formation are directly related to the tectonic environments in which they form. Indeed, plate tectonic interactions are fundamental to understanding geological processes. A new project funded by the National Science Foundation is focused on teaching […]
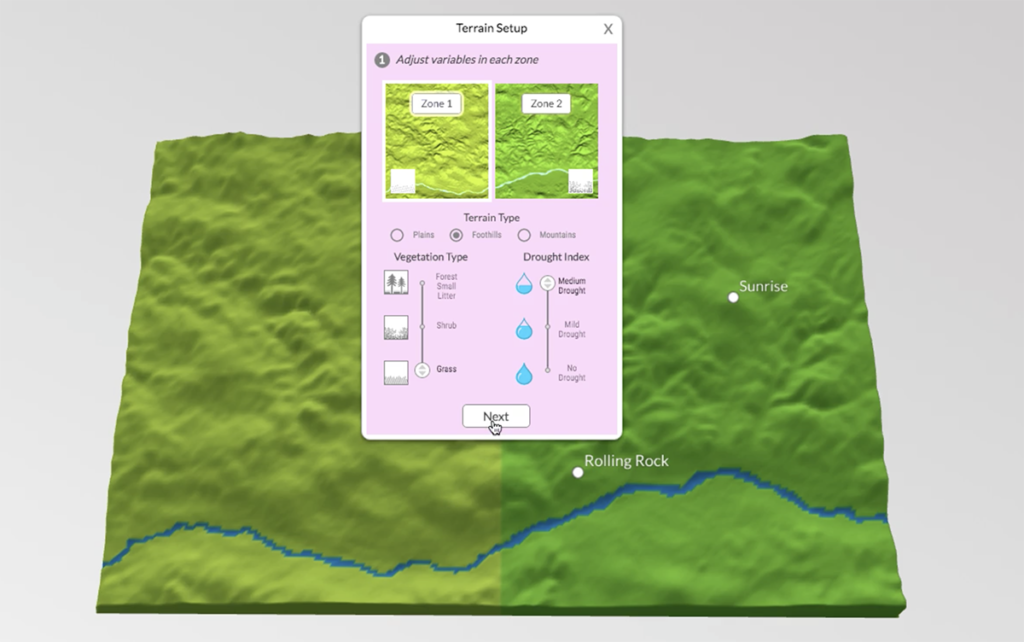
The GeoHazard team contributed to this blog post. We have just wrapped up a week of remote professional learning for our GeoHazard: Modeling Natural Hazards and Assessing Risks project. The goal of GeoHazard is to help students interpret data and understand the factors influencing the progression of and risks associated with natural hazards. Due to […]
Today marks the 50th anniversary of Earth Day. This holiday is devoted to our amazing planet and the resources we rely on. It is traditionally a day of environmentalism, devoted to sustainability and minimizing human impact on the environment. Earth Day 2020 comes in the midst of the COVID-19 pandemic that has radically changed our […]
All volcanic eruptions are dangerous. Some are more dangerous than others. Volcanic eruptions range from slow, relatively gentle flows of lava to explosive eruptions of gases, ash, and rock. Our Visualizing Geohazards and Risk with Code project (GeoCode) challenges students to model tephra volcanic eruptions (tephra refers to all particles ejected explosively from a volcano, […]
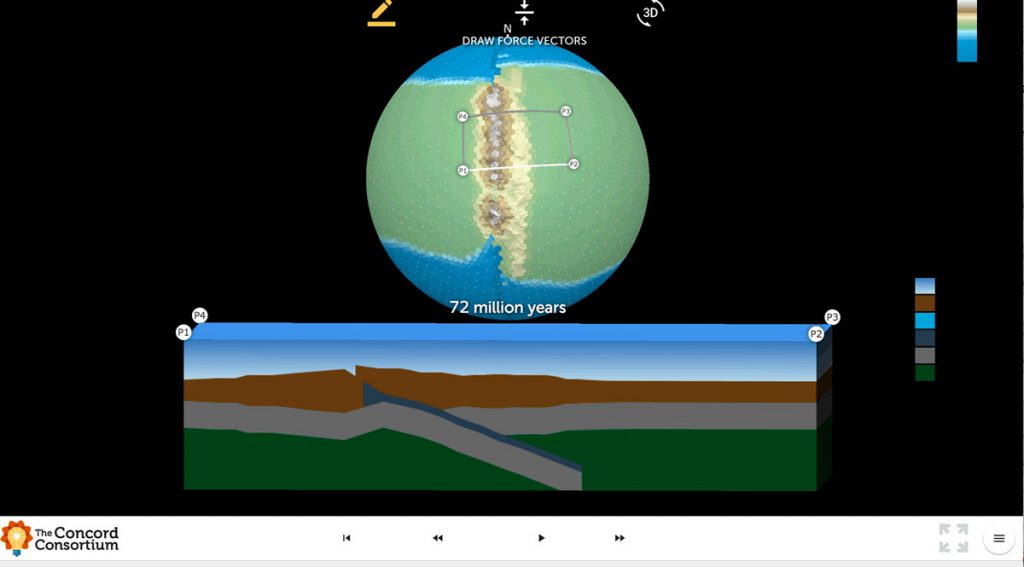
We are excited to introduce the *beta version* of Tectonic Explorer, our newest Earth system model, developed by our GEODE project. Tectonic Explorer features a complex system of interacting tectonic plates around an entire planet — in this case a simplified, Earth-like planet. For the first time in K-12 education, students will be able to […]